Poster
Investigation of Rock Origin by Water Chemistry Analysis
Alessandra Pistoia DePauw UniversityIntroduction
Geology influences water chemistry. As groundwater moves through a rock, it picks up the chemical signature of the rock. This chemical signature allows for the identification of rock that the water has travelled through, even when the water has travelled a good distance from this rock. Using this basic concept, I chose to analyse ocean water samples from the Southern coast of Kauai to investigate what type of rock water flows through. Basaltic rock accounts for a majority of the island, given its volcanic origin, however carbonate rock is also present. Water that flows through basaltic rock, carbonate rock, and simply seawater should carry different cations and anions, allowing us to differentiate between all three.
Not only can the chemistry of water inform us of its origin, but even more simply, it can tell us if it is freshwater or saltwater. This is helpful for identifying freshwater plumes in the ocean. These areas are of interest because it could indicate where groundwater is entering into the ocean. We are able to distinguish between saltwater and freshwater because saltwater has a higher concentration of sodium and chloride relative to freshwater.
Methods
Collection
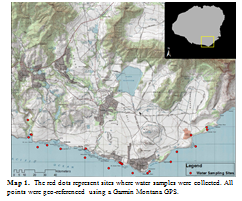
Twenty-three water samples were collected from the Southern coast Kauai, Hawaii during a two week period in mid-June (Map 1). Twelve samples were collected off-shore while aboard a fishing boat; all of these samples were collected within 550 meters of the shoreline. A temperature probe was used to monitor the fluctuation of temperature as we cruised across the ocean. There is a measurable distinction between freshwater and saltwater temperature, so in order to get samples of both, I collected a sample of water every time the temperature changed by at least one degree. Nine samples were collected near-shore either by snorkelling or walking a transect, whichever the turbulent ocean permitted; all samples were collected within 50 meters of the shoreline. Samples were collected in a similar, but not as quantitatively accurate, method as above: qualitative feeling was used to determine when the temperature changed. The last two samples were collected inland from springs. Since we assume that the water only travels through the rock type of the spring, these samples were used as a chemical signature baseline for volcanic rock and carbonate rock.
Analysis
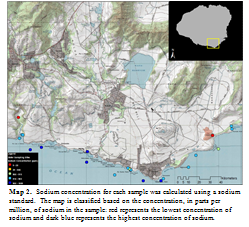
I filtered all samples using a 0.45 µm PTFE membrane with polypropylene housing. Then, 200 µL of each sample was placed into a vial and then diluted with 5 mL of deionized water; two identical vials were created for each water sample. These vials were placed into a Thermo Scientific Dionex IonPac, an ion chromatography (IC) machine; one vial was inserted into the AS11-HC Hydroxide-Selective Anion Exchange Column to measure anions and the other was inserted into the CS12A Analytical Column to measure cations. Chromeleon 7 was used to capture the information from the chromatograph and then to further analyse the data. Further analysis of the sodium ion was executed in order to distinguish between freshwater and saltwater. Dilutions of a sodium standard were processed through the IC at the same time as the other samples. Data from the standard was used to calculate concentrations of sodium in each of the samples. Concentrations of sodium were classified onto a map using ArcMap 10, to visually display the data (Map 2).
Data


Conclusions
Our data shows that we collected a lot of seawater samples. The largest ions found in all water samples were sodium and chloride (Figure 2 and Figure 4); these are the main ions that compose seawater. Given our sample set, we were unable to identify the rock type, with the exception of the two samples from the springs. We were also unable to identify any freshwater plumes from chemical analysis. We did find a higher concentration of nitrite and calcium in the spring samples, as compared to the seawater samples. Nitrite can be derived form organic matter and calcium can be derived from precipitation of limestone.
Although no great conclusions can be drawn from this data, it is a beneficial to know that ion chromatography is a very powerful way to measure ion concentrations in water samples. Given the time constraints of the program and the unfamiliarity with the machine and the software associated with the machine, I was unable to get the machine to read all ions correctly. Some of the ion standards that I used were not always picked up by the machine, which makes me question the analysis. Sodium was one of the few that seemed to be working properly. Although sodium does not tell us much more about the water other than how salt it is, I mapped sodium concentrations because the standard confirmed that the machine was reading it properly. The map is of no great importance, but it is an example of how effective mapping can be to visually display results of ion chromatography.
In the future, this similar analytical methods can be used to identify and map other, more telling ions. For example, silica is another ion that should be considered for distinguishing between freshwater and saltwater, especially in the Hawaiian Islands. Volcanic rock has high concentrations of silica, thus water will pick up a chemical signature that mimics this high concentration. This may lead to a discovery of freshwater plumes in the ocean.
Acknowledgements
Many thanks to Dr. Becker, Weston Ellis, Courtney Fiamengo, Alison Yoho, Kelly Acridge, Matthew Lucas, GRAM REU, Nation Tropical Botanical Garden, NSF, CSULB, and DePauw University.
References
Fetter, C. W. Applied Hydrogeology. Upper Saddle River, NJ: Prentice Hall, 2001. Print.
